CAS 54965-24-1
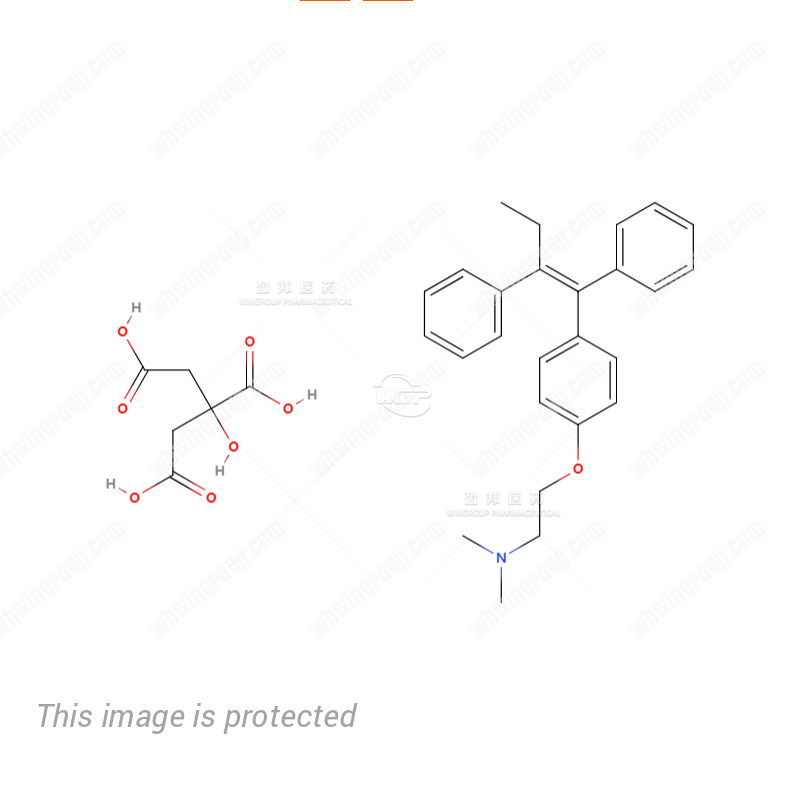
MF: C32H37NO8
MW: 563.64
Tamoxifen Citrate is a selective estrogen response modifier (SERM), protein kinase C inhibitor and anti-angiogenetic factor. Tamoxifen is a prodrug that is metabolized to active metabolites 4-hydroxytamoxifen (4-OHT) and endoxifen by cytochrome P450 isoforms CYP2D6 and CYP3A4.
- Description
- Additional information
Description
Tamoxifen citrate CAS 54965-24-1 Product Information
| Product Name: | Tamoxifen citrate |
| Synonyms: | (Z)-[2-[4-(1,2-diphenylbut-1-enyl)phenoxy]ethyl]dimethylammonium dihydrogen 2-hydroxypropane-1,2,3-tricarboxylate;TAMOXIPHEN;Ethanamine, 2-4-(1Z)-1,2-diphenyl-1-butenylphenoxy-N,N-dimethyl-, 2-hydroxy-1,2,3-propanetricarboxylate (1:1);TAMOXIFENCITRATE,USP;TAMOXIFEN CITRATE ANTI-ESTROGEN, PROTEI;TomixipheneCitrate;(Z)-1-(4-Dimethylaminoethoxyphenyl)-1,2-diphenyl-1-butene Citrate;(Z)-2-(4-(1,2-Diphenyl-1-butenyl)phenoxy)-N,N-dimethylethanamine, citrate (1:1) |
| CAS NO: | 54965-24-1 |
| Molecular Weight: | 563.64 |
| Molecular Formula: | C32H37NO8 |
| Melting point | 140-144 °C |
| solubility: | methanol: soluble50mg/mL, clear, colorless |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20° for up to 2 months. |
| Appearance: | White to off-white Powder |
tamoxifen citrate bodybuilding
Tamoxifen citrate is a selective estrogen receptor modulator (SERM) that is primarily used in the treatment and prevention of breast cancer. It works by blocking the effects of estrogen in breast tissue, which can help prevent the growth and spread of breast cancer cells.
In the context of bodybuilding, tamoxifen citrate is sometimes used by individuals who are taking anabolic steroids. Anabolic steroids can increase the levels of estrogen in the body, leading to side effects such as gynecomastia (enlargement of breast tissue in males). Tamoxifen citrate is used to counteract these estrogenic side effects by blocking the estrogen receptors in breast tissue.
It’s important to note that the use of tamoxifen citrate for bodybuilding purposes is considered off-label and is not approved by regulatory authorities for this use. Additionally, tamoxifen citrate should only be used under the guidance of a healthcare professional, as it can have potential side effects and interactions with other medications.
If you are considering using tamoxifen citrate or any other medications for bodybuilding purposes, it’s important to consult with a healthcare professional who can provide appropriate guidance, monitor your health, and ensure your safety.
tamoxifen citrate pct
Tamoxifen citrate is commonly used in post-cycle therapy (PCT) by individuals who have completed a cycle of anabolic steroids or other performance-enhancing substances. PCT is a period of time following the use of these compounds where individuals aim to restore their natural hormone production and minimize the potential side effects associated with discontinuing steroid use.
During PCT, tamoxifen citrate is used to help restore the body’s natural testosterone production. Anabolic steroids can suppress the body’s own production of testosterone, and tamoxifen citrate can stimulate the release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) from the pituitary gland. This, in turn, can promote the production of testosterone in the testes.
Typically, tamoxifen citrate is administered at a dosage of 20-40 mg per day for several weeks during PCT. The exact duration and dosage may vary depending on the specific steroid cycle and individual needs. It’s important to note that PCT protocols can differ, and it’s recommended to consult with a healthcare professional or a knowledgeable expert in the field to develop a personalized plan tailored to your circumstances.
It’s worth emphasizing that tamoxifen citrate should only be used under the guidance of a healthcare professional. They can monitor your hormone levels, provide appropriate guidance, and help manage any potential side effects or interactions with other medications.
We are Chinese professional supplier of tamoxifen citrate for sale,Inquiries are always welcome.
tamoxifen citrate side effects
- Menopause-like symptoms, including hot flashes, night sweats and vaginal dryness.
- Weight gain (more common) or fluid retention (edema).
- Irregular or loss of menstrual periods.
- Leg swelling.
- Nausea.
- Vaginal discharge.
- Skin rash.
- Erectile dysfunction.
tamoxifen citrate for males
Most male breast cancers are hormone-dependent, so estrogen-blocking treatments including Tamoxifen are often used. Possible side effects for men taking Tamoxifen include headaches, nausea, hot flashes, skin rash, fatigue, sexual dysfunction, and weight and mood changes.
Additional information
| Color | White |
|---|---|
| Form | Powder |







Reviews
There are no reviews yet.