Ethyl pyruvate
CAS:617-35-6
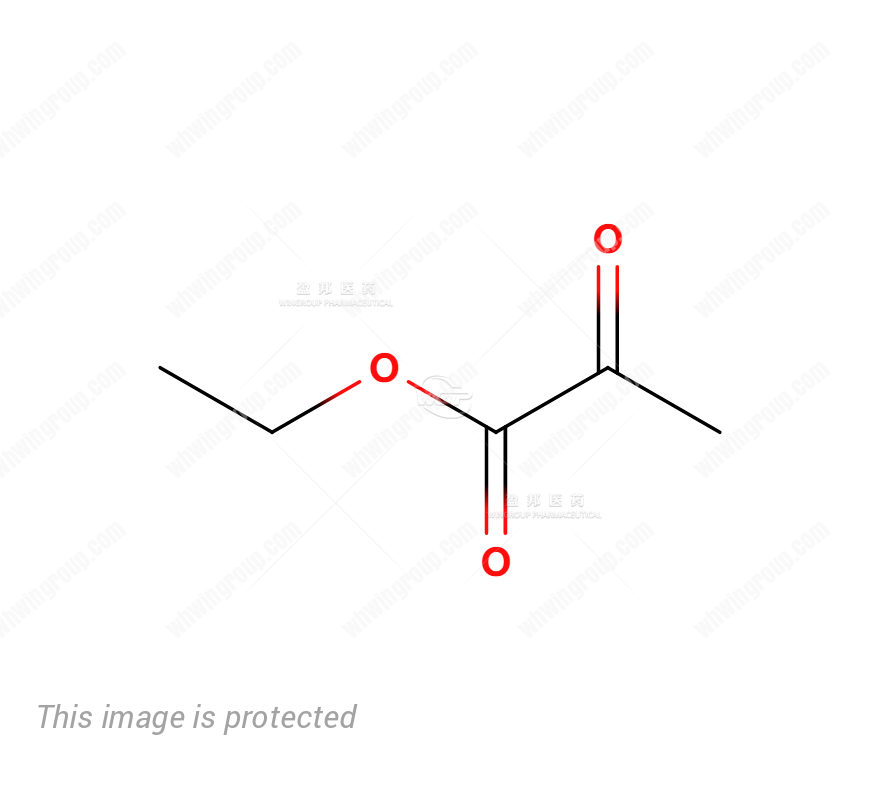
MF:
It can be used in the manufacture of pharmaceutical pindolol and pesticides thiabendazole. At the same time, it is an important intermediate in the pharmaceutical and chemical industry.
- Description
- Additional information
Description
Ethyl pyruvate CAS 617-35-6 Product Information
| Product Name: | Ethyl pyruvate |
| Synonyms: | PYR ET;RARECHEM AL BI 0116;ETHYL PYRUVATE;FEMA 2457;Ethyl methylglyoxylate;2-Oxopropionic acid ethyl;Pyruvic acid ethyl;NSC 48386 |
| CAS NO: | 617-35-6 |
| Molecular Weight: | 116.12 |
| Molecular Formula: | C5H8O3 |
| Boiling Point: | 155.0±0.0 °C at 760 mmHg |
| Melting point: | -58 °C |
| Density: | 1.0±0.1 g/cm3 |
| Appearance: | Clear pale yellow Liquid |
| Applications: | It can be used in the manufacture of pharmaceutical pindolol and pesticides thiabendazole. At the same time, it is an important intermediate of pharmaceutical and chemical industry. |
| Solubility: | Miscible with water, ethanol and ether. |
| Storage: | Store at +2°C to +8°C. |
ethyl pyruvate uses
t is widely applied to various kinds of industries including medicine, pesticides, flavors and fragrances, food additives, air fresheners, fine chemicals and other industries. It can be used in the synthesis of cardiovascular drugs like expansion agent; it can also be applied to cosmetic of skin whitening and nutrition and can promote the healing of the wound, prevention and treatment of skin disease characterized by cracking, spalling and squamous metaplasia; it can be used as the high-efficiency active ingredients in the air fresheners and can effectively eliminate the ammonia and methyl mercaptan in the air; because itself has a special flavor, it can be applied to flavors, fragrance; it is also an important raw material for synthetic resins and plastics.
conversion of pyruvic acid to ethyl alcohol/What is the process by which pyruvic acid is converted to ethyl alcolohol?
In the first step, pyruvate is decarboxylated in an irreversible reaction catalyzed by pyruvate decarboxylase. Pyruvate decarboxylase requires Mg and has a tightly bound coenzyme, thiamine pyrophosphate.
In the second step, acetaldehyde is reduced to ethanol through the action of alcohol dehydrogenase, with the reducing power furnished by NADH derived from the dehydrogenation of glyceraldehyde 3-phosphate.
Ethanol and CO are thus the end products of ethanol fermentation, and the overall equation is as follows:
Glucose + 2ADP + 2Pi —-> 2 ethanol + 2 CO + 2 ATP + 2 HO
ethyl pyruvate solubility
Miscible with water, ethanol and ether.
ethyl pyruvate structure
Ethyl pyruvate belongs to the class of organic compounds known as enoate esters. These are an alpha,beta-unsaturated carboxylic ester of general formula R1C(R2)=C(R3)C(=O)OR4 (R4= organyl compound) in which the ester C=O. function is conjugated to a C=C double bond at the alpha, beta position.
ethyl pyruvate synthesis
Ethyl pyruvate a derivative of the metabolite pyruvic acid, can be synthesized from pyruvic acid and ethanol via esterification reaction in a water-free or water-restricted solvent system.
ethyl pyruvate melting point
-58 °C

Additional information
| Melting Point | -58 °C |
|---|---|
| Boiling point | 144 °C (lit.) |
| Density | 1.045 g/mL at 25 °C (lit.) |
| Solubility | 10g/l |
| Color | Colorless |
| Form | Liquid |












Reviews
There are no reviews yet.